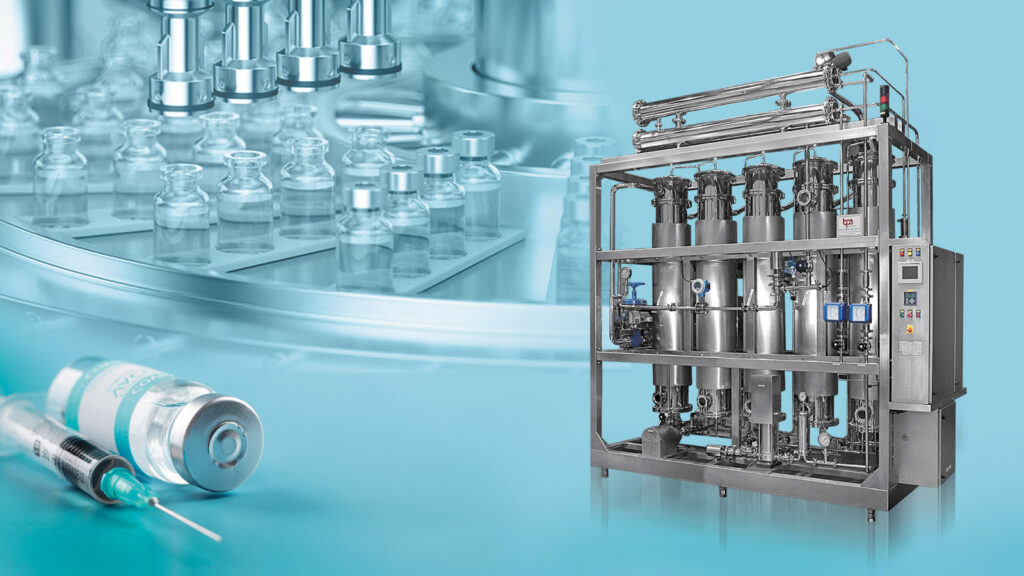
Multi Column Distillation (WFI) Plant
- August 6, 2023
- Bright Pharma Engg Pvt Ltd
Bright Pharma Engineering is an advanced equipment manufacturing company specializing in the production of process equipment for the pharmaceutical, bio-pharma, healthcare, and cosmetic industries. We undertake the production of aseptic mixing vessels based on the ASME BPE guidelines, equipped with aseptic magnetic mixers and advanced instruments as per EHEDG guidelines. Our CIP modules come in a variety of types and are custom-built to clean the vessels. We understand that the cleaning of production equipment is an extremely essential part of the pharmaceutical production process, and thus it holds equal importance to the production process itself, according to FDA guidelines. The cleaning of all equipment must adhere to strict standards of documentation, and the process must be validated based on the standards laid down by the FDA. At Bright Pharma Engineering, we have a state-of-the-art system that delivers WFI of a higher quality standard for storage and distribution, surpassing the standards imposed by pharmacopeias worldwide.
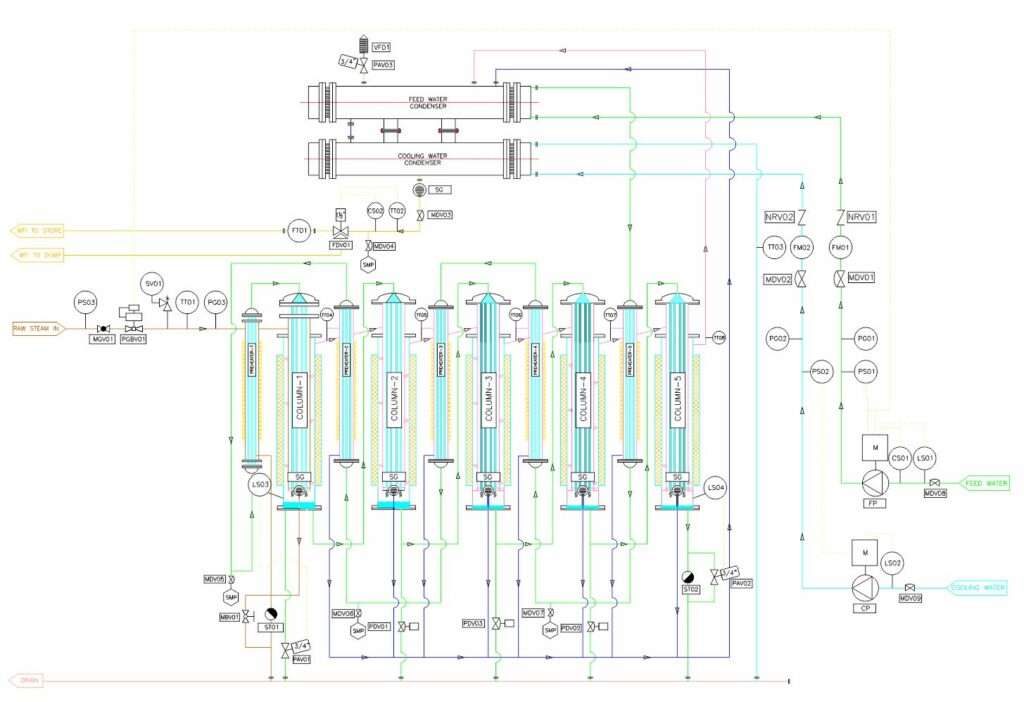
The multiple-column distillation plant is designed and manufactured in accordance with the ASME Sec VIII Div & TEMA fabrication code. These columns are used to produce pure steam in the first column. The steam is then condensed and further distilled in the following columns. This design helps to lower operational expenses and results in the production of high-quality Water for Injection (WFI).
How It Works
Each column has a capacity ranging from 50 to 5000 liters/hour and employs double tube sheet construction for feed water, cooling water, and condenser. Condensation occurs using a thin-falling film technology, which is repeated in each column. Increasing the number of columns decreases the overall equipment consumption. However, the quantity of columns does not affect the quality or output of the equipment. Additionally, each column is equipped with a special labyrinth separator at the top to separate the steam generated during evaporation from any entrained substances. The result is pure, “dry,” and pyrogen-free steam, which is then condensed into compendial WFI.
Applications
A sophisticated industrial system is used for the production of high-purity water or distilled water. It employs a series of distillation columns to remove impurities and contaminants from the feed water, resulting in a purified liquid product. The multi-column distillation plant finds application in various industries for high-quality water is a critical requirement.
- Pharmaceutical Industry – In the pharma industry, the production of pure and sterile water is of utmost importance. This plant ensures the removal of impurities, such as dissolved solids, organic compounds, etc from the water used for drug formulation, injection solutions, and sterile manufacturing processes. It meets the stringent water quality standards required for pharmaceutical production and ensures the safety and efficacy of pharmaceutical products.
- Biotechnology and Life Sciences – The WFI plant plays a vital role in biotechnology and life sciences research and manufacturing processes. The plant ensures the elimination of contaminants that could interfere with critical biological processes, maintaining the integrity and reliability of experimental results and product quality.
- Food and Beverage Industry – The food and beverage industry relies on purified water for a wide range of applications, such as ingredient preparation, beverage production, and equipment cleaning. A WFI distillation plant ensures the removal of impurities, including minerals, dissolved solids, and potential contaminants. It helps maintain the desired taste, texture, and shelf life of various food and beverage items.
- Laboratories and Research Facilities – High-quality water is often required for Research laboratories, scientific institutions, testing facilities experimental procedures, analytical equipment, and sample preparation. The multi-column distillation plant provides reliable purified water, to meet the stringent standards for research & analysis.

